The US Centres for Disease Control and Prevention (CDC) has linked four Indian-made cough syrups contaminated with diethylene glycol (DEG) with the deaths of 66 children in The Gambia from acute kidney injury last year.
The cough syrups were manufactured by an Indian pharmaceutical firm based in Haryana and were imported to The Gambia in 2022.
In its Morbidity and Mortality report, dated March 3, the CDC stated, “The implicated syrup-based paediatric medications that were administered to patients were imported from a single Indian manufacturer.
“This is one of the first documented DEG outbreaks in which contaminated medications were imported rather than being domestically manufactured.”
AKI outbreaks associated with DEG contamination have been documented earlier in Panama, Nigeria, India and Haiti, said the report, adding that in the past incidents, “manufacturers have been suspected of substituting DEG for more expensive, pharmaceutical-grade solvents”.
The report pointed out that inadequate regulatory structures made the sale of medications from international markets an especially high-risk activity in low-resource settings.
“Medications for export might be subject to less rigorous regulatory standards than those for domestic use. Simultaneously, low-resource countries might not have the human and financial resources to monitor and test imported drugs,” the report stated.
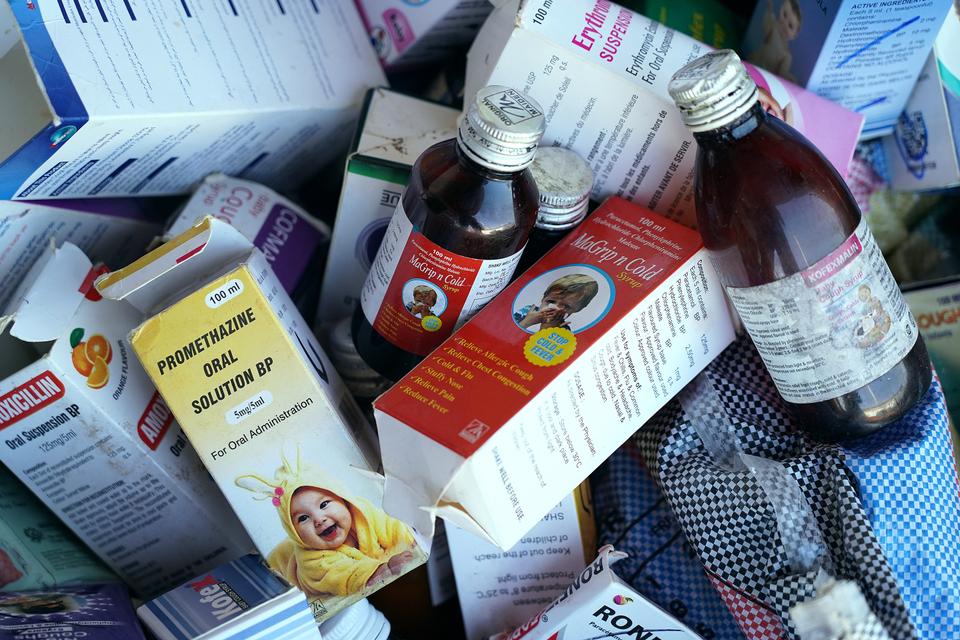
The WHO identified the medicines as Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup.
The four products had been identified in The Gambia, but “may have been distributed, through informal markets, to other countries or regions”, the WHO added, in the alert published on its website.
It warned that their use may result in serious injury or death, especially among children.
The WHO’s intervention came after medical authorities in The Gambia detected an increase in cases of acute kidney injury among children under the age of five in late July.










Recent Comments